Thinking Out of the Box: Removing Medical Devices with a Liquid Metal
By Helen Wong 王思齊

What happens when several drops of the liquid metal gallium are added to an aluminum can? Our high school chemistry class taught us that nothing would happen. But if you wait for a while, you will be surprised to see the can shatters into pieces with just a single touch.
Are our chemistry teachers wrong? No. The shattering of aluminum cans upon exposure to gallium is not caused by a chemical reaction. Instead, it is the result of a physical phenomenon known as liquid metal embrittlement (LME).
Although a metal object (say an aluminum can) may appear as a single piece, it actually consists of many small crystals called grains. As shown in Figure 1, when the can comes into contact with specific liquid metals like gallium, the latter can penetrate the boundaries, or spaces, between the grains [1]. This significantly weakens the cohesion of the grains and hence the strength of the aluminum can, making it susceptible to fracture.
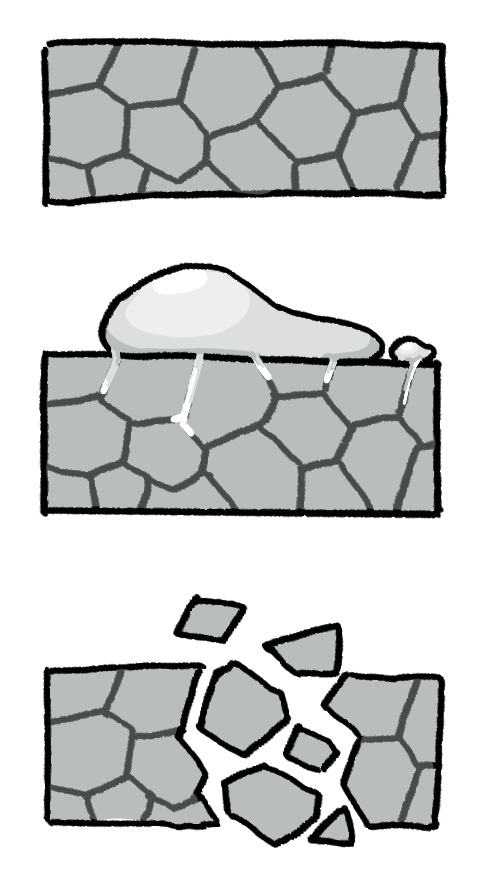
Figure 1 The process of liquid metal embrittlement (LME) [2].
While LME has been a common source of metal structure failure in industries such as aerospace and construction, a group of researchers at the Massachusetts Institute of Technology recently “harnessed this failure mechanism in a productive way [2, 3].”
Metals have properties ideal for making biomedical devices: They are strong, durable, and have excellent electrical and thermal conductivity. However, a major problem when using metal devices is the way to remove them when they are not required anymore. This can possibly be done by surgery or endoscopy (footnote 1), yet these invasive procedures may cause additional tissue damage. Therefore, the researchers started exploring devices that can disintegrate inside the patient’s body after use.
Drawing inspiration from LME, the research team experimented on the use of a gallium alloy called eutectic gallium-indium (EGaIn) for the dissolution of different aluminum devices. Gallium stands out from other LME-inducing liquid metals for two reasons. First, it can prevent the formation of a surface oxide layer on the aluminum device upon application. This allows aluminum to react with water and enhances its degradation via dissolution. More importantly, gallium is biocompatible – acute toxicity studies showed EGaIn is non-toxic to rodents even at high doses.
The next step is to deliver gallium-indium to aluminum devices, either directly or indirectly. The former involves smearing EGaIn paint onto devices such as staples used to hold the skin together (Figure 2). This may appear trivial, but it is not an easy task. Like water, EGaIn has high surface tension that hinders its ability to attach to and spread over metal surfaces. Knowing that gallium oxide has a much lower surface tension, the researchers applied a simple trick – physically stirring EGaIn beforehand – to increase the alloy’s exposure to air and hence the ratio of gallium oxide to EGaIn in the paint. Alternatively, nano- and microparticles of EGaIn were produced for delivery into patients’ bodies to trigger the dissolution remotely. The team treated different biomedical devices made of aluminum, such as staples on skin and stents implanted in the esophagus, with EGaIn suspensions and found that these metal structures were broken down shortly afterward.
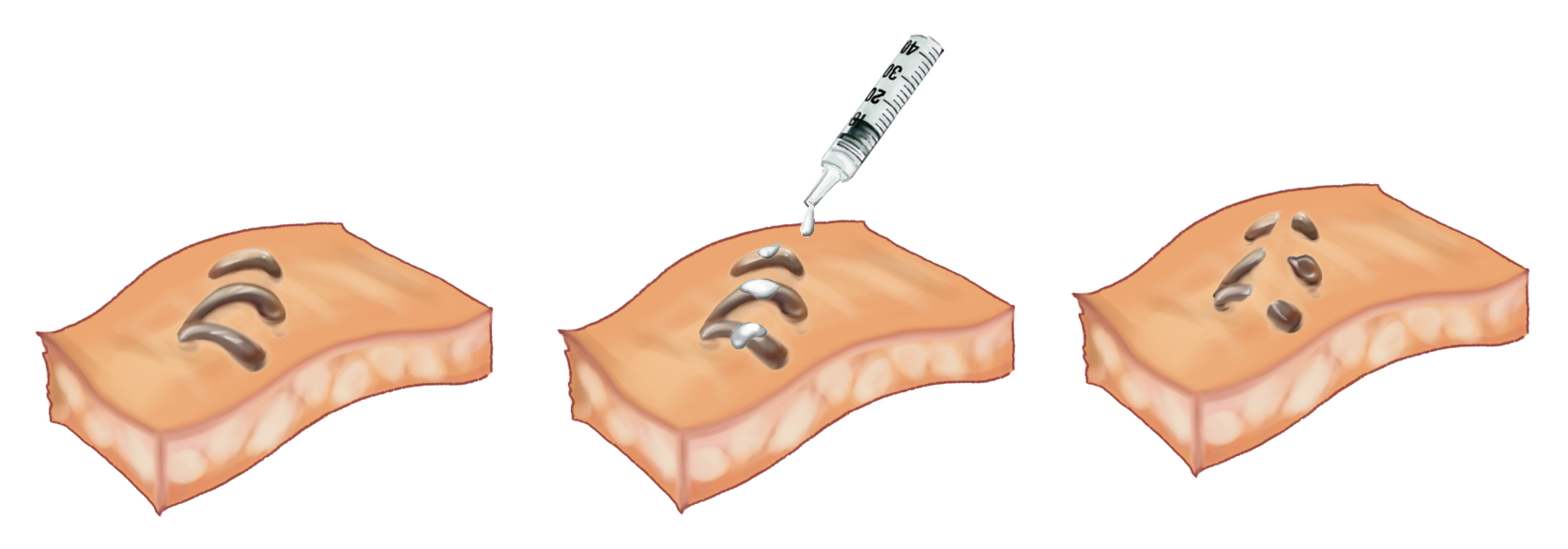
Figure 2 Smearing EGaIn paint onto a staple to remove the device.
Although gallium-induced embrittlement works well for aluminum devices, what about devices made of other metals? For instance, esophageal stents are often made of metals such as nitinol, a nickel-titanium alloy, instead of aluminum. To widen the applicability of LME in the removal of biomedical devices, the researchers have also been exploring the possibility of creating dissolvable devices made of nitinol and other metals commonly used in medical settings.
It may take some time before these dissolvable metal devices are ready for clinical use, but the genuine creativity demonstrated in this study is immediately apparent. While most people perceive LME as a failure mechanism, the researchers thought out of the box to turn such a mechanism into a productive one. At times, good research does not require highly sophisticated methods; a touch of creativity can make all the difference.
1 Editor’s note: In addition to the camera to look inside the body, various tools can be attached to the tip of the endoscope, such as grasping forceps (for retrieving foreign objects), and biopsy forceps (for performing biopsies).
References:
[1] Norkett JE, Dickey MD, Miller VM. A review of liquid metal embrittlement: Cracking open the disparate mechanisms. Metallurgical and Materials Transactions A. 2021;52:2158-2172. doi:10.1007/s11661-021-06256-y
[2] Feig VR, Remlova E, Muller B, et al. Actively triggerable metals via liquid metal embrittlement for biomedical applications. Advanced Materials. 2022;35(11). doi:10.1002/adma.202208227
[3] Trafton A. An easier way to remove medical devices. MIT News. https://news.mit.edu/2022/medical-devices-aluminum-1108.