Thinking Out of the Box: Oral Insulin Pill
By Helen Wong 王思齊
Imagine the following scenario: You are required to stab your abdomen with a needle up to four times a day and seven days a week. Wouldn’t this be a nightmare for you? This is exactly what happens to people with type I diabetes [1]. These patients need regular insulin injections as their bodies cannot produce enough insulin to effectively lower blood glucose levels.
To liberate type I diabetes patients from the nightmare, scientists and doctors have been searching for reliable oral insulin delivery systems for decades. However, extreme pH, the presence of proteases and thick mucus layers along the gastrointestinal (GI) tract posed great challenges to such a quest.
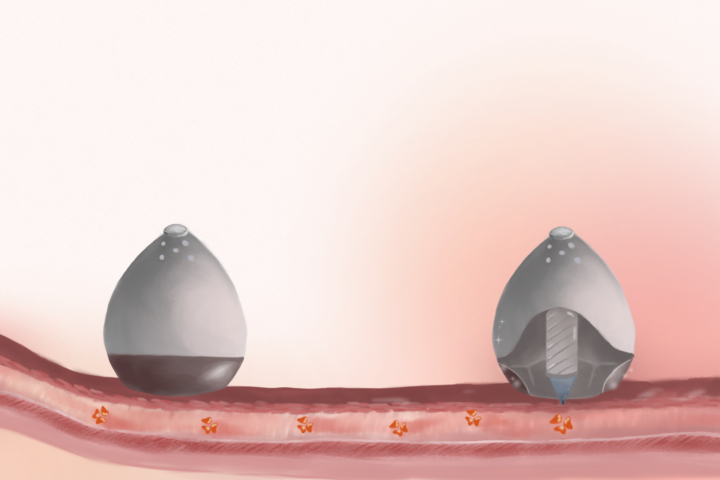
In 2019, researchers at the Massachusetts Institute of Technology reported a breakthrough study in which they designed a novel ingestible device called self-orienting millimeter-scale applicator (SOMA) only at the size of a blueberry [2, 3]. While the concept of SOMA is simple – a capsule that contains a hidden needle, which would inject insulin once the device is in contact with the stomach wall, it has a well-thought-out design. Let’s take a closer look at the challenging but successful journey of SOMA along the GI tract!
After being swallowed, a SOMA would pass through the esophagus and enter the stomach. As the first step, the capsule would localize and self-orient its injection mechanism toward the stomach lining. This ingenious self-orientation system was inspired by the seemingly unrelated leopard tortoise (Stigmochelys paradalis). This tortoise species has a shifted center of mass and a steep-dome-shaped shell that allow it to always self-orient to a preferred upright position. Researchers applied computer models to optimize SOMA’s dome shape, and used a combination of low-density polycaprolactone and high-density stainless steel to lower its center of mass. Experiments showed that SOMA self-orientation only took one second.
The second step is insulin injection. The needle inside the capsule has two components: a tip made of compressed insulin and a biodegradable shaft. Only the dissolvable insulin tip would pierce the stomach wall. To provide enough force for needle insertion into the stomach wall, the team further attached a compressed spring, which was initially fixed in sucrose and isomalt, to the needle. During actuation, water in the stomach would enter SOMA through its vents to dissolve the sugar barrier. This would release the spring and insulin would be injected into the stomach wall. The whole process required one minute and subsequent dissolution of the solid insulin drug lasted for about an hour under experimental conditions. After successful insulin delivery, the capsule would pass through the remainder of the GI tract and eventually be excreted in feces.
“The design of SOMA sounds cool, but what’s the point of injecting insulin into the stomach wall instead of the abdomen? Needle injection is always painful!” You may have the same question in mind as you are reading through this article. Please rest assured that patients would not feel any pain as the stomach wall has no pain receptors. The effectiveness of stomach wall injection by SOMA was also proved to be comparable to conventional subcutaneous injection in pig studies.
In fact, insulin is just one of the many drugs that could potentially be delivered by SOMA. To improve the applicability of SOMA, the team reported a new version of SOMA in 2021, the liquid-injecting SOMA (L-SOMA). It could deliver monoclonal antibodies in liquid form and at larger dosing volumes [4], implying that it could potentially target cancers and autoimmune diseases (e.g. rheumatoid arthritis) besides diabetes. The scope of applicable biomacromolecules further expanded to RNA in early 2022 [5, 6]. As single-stranded RNA is susceptible to degradation, researchers made use of protective polymeric nanoparticles to produce RNA-nanoparticle complexes. Results showed that SOMA was able to deliver these complexes into stomach cells at an amount comparable to that in COVID-19 vaccines. We are a step closer to oral mRNA vaccines now!
References:
[1] American Diabetes Association. Insulin Routines. https://diabetes.org/healthy-living/medication-treatments/insulin-other-injectables/insulin-routines.
[2] Abramson A, Caffarel-Salvador E, Khang M, et al. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019;363(6427):611-615. doi:10.1126/science.aau2277
[3] Trafton A. New Pill can deliver insulin. MIT News. https://news.mit.edu/2019/pill-deliver-insulin-orally-0207.
[4] Abramson A, Frederiksen MR, Vegge A, et al. Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nature Biotechnology. 2021;40(1):103-109. doi:10.1038/s41587-021-01024-0
[5] Abramson A, Kirtane AR, Shi Y, et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter. 2022;5(3):975-987. doi:10.1016/j.matt.2021.12.022
[6] Trafton A. Making RNA vaccines easier to swallow. MIT News. https://news.mit.edu/2022/oral-rna-vaccines-0131.