The Science of Fried Chicken
By Michelle Lo 盧慧敏 (Yew Chung International School)

Who doesn’t love a piece of crunchy, crispy fried chicken? Invented in Scotland and developed in the American South, fried chicken has evolved over the centuries to become a fast food staple in our modern lives. However, a surprising amount of chemistry goes into making your finger-lickin’ good.
In fact, the mouth-watering flavour and texture of fried chicken can be attributed to various chemical processes, including partial dissociation of the rigid microscopic structure of muscle, enzyme catabolism, and the Maillard reaction.
The first chemical process occurs before frying, when the chicken is marinated through brining. The chloride ions (Cl–) in the brine (which is scientifically called sodium chloride solution) cause disruption to the tight packing of the muscle filaments (see “thin filaments” and “thick filaments” in Figure 1) [1, 2]. The negatively charged chloride ions work by binding to the muscle filaments and increase the electrostatic repulsive force between them [2]. The rigid, regular microscopic repeating unit of muscle, namely sarcomere, which forms the muscle fibrils, is therefore partially dissociated (Figure 1, 2). Without the complete, well-organised structure of sarcomere, the muscular tissue can accommodate more water content before cooking [2, 3], and does not tighten up to squeeze out the liquid during heating [1, 3]. Hence, more moisture can be retained and the meat can remain juicy.

Figure 1. Sarcomere is the repeating unit which forms muscle fibril (myofibril). Chloride ions work by disrupting the regular, rigid structure of sarcomere. (Photo credit: CFCF)
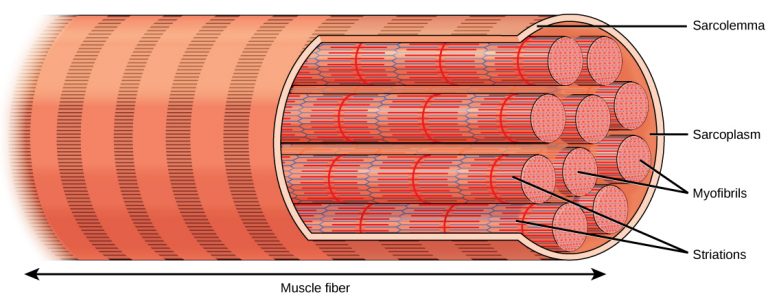
Figure 2. Muscle fibre (myofibre), which is a larger structure, consists of a bunch of muscle fibrils. (Photo credit: CFCF)
After brining, the chicken is coated in a batter of flour, spices, and buttermilk. Aside from helping the coating stick to the skin, the buttermilk also contains enzymes that digest proteins in the chicken. This makes the slightly acidic buttermilk perfect for further tenderising the meat [4].
Finally, the chicken is immersed in hot oil at temperatures of around 150 – 190°C. This is when a series of chemical reactions – called the Maillard reaction – occur. During this process, heat causes the reactive carbonyl group1 of sugar to react with nucleophilic amino acids, eventually producing hundreds of small colour and flavour compounds of great complexity [5]. The reaction therefore contributes to the golden colour and the irresistible aroma of the crunchy crust – which is not only good for munching on, but is also good for inhibiting excessive oil absorption after frying.
However, aside from the chicken, the oil used for deep frying also experiences chemical changes – namely in the form of hydrolysis, oxidation, and polymerisation.
Hydrolysis is the chemical reaction in which water breaks down the bonds of a particular compound. During frying, water escapes the chicken in the form of rapid-fire bubbles. In addition to forming a protective steam barrier against the oil, these water bubbles break down the ester linkage of triacylglycerols in the oil and produce smaller acylglycerols, glycerol, and free fatty acids [6].
These free fatty acids continue to play a key part in oxidation. When certain free fatty acids such as linoleic acid come into contact with air, they oxidise and form smelly volatile compounds like ketones, alkanes, aldehydes, carboxylic acids, and alkenes. The heat of the deep fryer also causes the polymerization of fatty acids, as the linked fatty acid molecules form nonvolatile polar compounds and polymers, which all contribute to the deterioration of oil during cooking [7].
Hence, if the oil is not replaced, these compounds can build up in the deep fryer and give the chicken an unpleasant fishy flavour. That’s why fast food workers have to keep changing the oil in their frying machines, in order to give you the perfect piece of poultry.
So, next time you order fried chicken from your favorite fast food restaurant, you may want to take some time to appreciate all the science that went into making your delicious food.
1 Carbonyl group is a functional group composed of C=O. Aldehydes (RCHO), ketones (R(C=O)R’) and carboxylic acids (RCOOH) are also compounds which contain a carbonyl group.
References
[1] Kenji López-Alt, James. “The Best Southern Fried Chicken.” The Food Lab, Serious Eats, July 2015. https://www.seriouseats.com/2015/07/the-food-lab-best-southern-fried-chicken.html
[2] EDinformatics. “How Does Brining Work?”. EDinformatics, n.d. http://www.edinformatics.com/math_science/science_of_cooking/brining.htm
[3] Kenji López-Alt, James. “The Food Lab: The Truth About Brining Turkey.” The Food Lab, Serious Eats, November 2012. https://www.seriouseats.com/2012/11/the-food-lab-the-truth-about-brining-turkey-thanksgiving.html
[4] Yummy Team. “What Is Buttermilk and Why Do You Need it for Fried Chicken?” Yummy, 9 May 2017. https://www.yummy.ph/lessons/cooking/buttermilk-why-do-you-need-it-for-fried-chicken
[5] Hartings, Matthew. “Maillard Reaction.” Chemical & Engineering News, 12 December. https://cen.acs.org/articles/89/i47/Maillard-Reaction.html
[6] E. Choe and D.B. Min. “Chemistry of Deep-Fat Frying Oils.” Journal of Food Science, vol. 72, nr. 5, 2007. https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1750-3841.2007.00352.x
[7] Reactions. “The chemistry of fried chicken (video).” American Chemical Society, PBS Digital Studios, 20 July 2017. https://www.acs.org/content/acs/en/pressroom/newsreleases/2017/july/the-chemistry-of-fried-chicken-video.html