Getting Rid of Glasses: A Beginner's Guide to Contact Lenses
By Jane Yang 楊靜悠

Introduction
For those of you who wear glasses, you should have experienced the frustration on a scorching summer day. Can you recall the annoyance of sweat trickling down your temples, fogging up your lenses and obscuring your vision? During physical activities, your glasses constantly slip down your nose, interrupting your focus and hindering your performance. Besides, have you noticed how glasses can sometimes make your eyes appear smaller and less expressive?
Unfortunately, myopia, or short-sightedness, is a common problem among teenagers. In Hong Kong, the rate of myopia is about 18% in 6-year-old, and 62% in 12-year-old students [1]. While glasses are a common solution, if you're tired of being restricted by glasses, contact lenses are an alternative option.
The History of Contact Lenses
The prototype of contact lenses has been around for centuries. Leonardo da Vinci first conceived the idea of wearing a huge glass hemisphere filled with water in front of the eyes to correct eyesight in 1508 [2]. It was not until 1887 that we are technologically capable of creating the first glass lenses in direct contact with the cornea [3]. Unfortunately, these glass lenses were very uncomfortable and inconvenient to wear, and could only be worn for a few hours [4].
From the 1930s, the development of polymer chemistry provided a new option for producing contact lenses [3, 4]. Poly(methyl methacrylate) (PMMA) was the first polymer used to make thinner contact lenses with better clarity, flexibility, and lighter weight. Despite the huge improvement in comfort level, PMMA lenses were still rigid and considered uncomfortable to wear [4, 5].
Other than rigidity, contact lenses made of PMMA have a more serious drawback: The oxygen can barely pass through the lenses [4, 5]. Low oxygen transmissibility can cause complications like corneal swelling, corneal neovascularization (the formation of new blood vessels into the transparent cornea) and loss of corneal transparency [6]. Therefore it is important to choose a material with a high oxygen transmissibility [7].
Contact Lenses Today
Soft contact lenses made of hydrogel are the most common types of contact lenses used today [4]. Hydrogel consists of a hydrophilic (water-loving) but insoluble cross-linked polymer network (see Figure 1). Due to the highly electronegative atoms such as oxygen atoms in the polymer, water molecules can be trapped in the network to create a soft, flexible jelly-like structure by forming hydrogen bonds with the electronegative atoms in the structure. As a result, the material can absorb as much as 85 to 90 percent of water by weight [3].
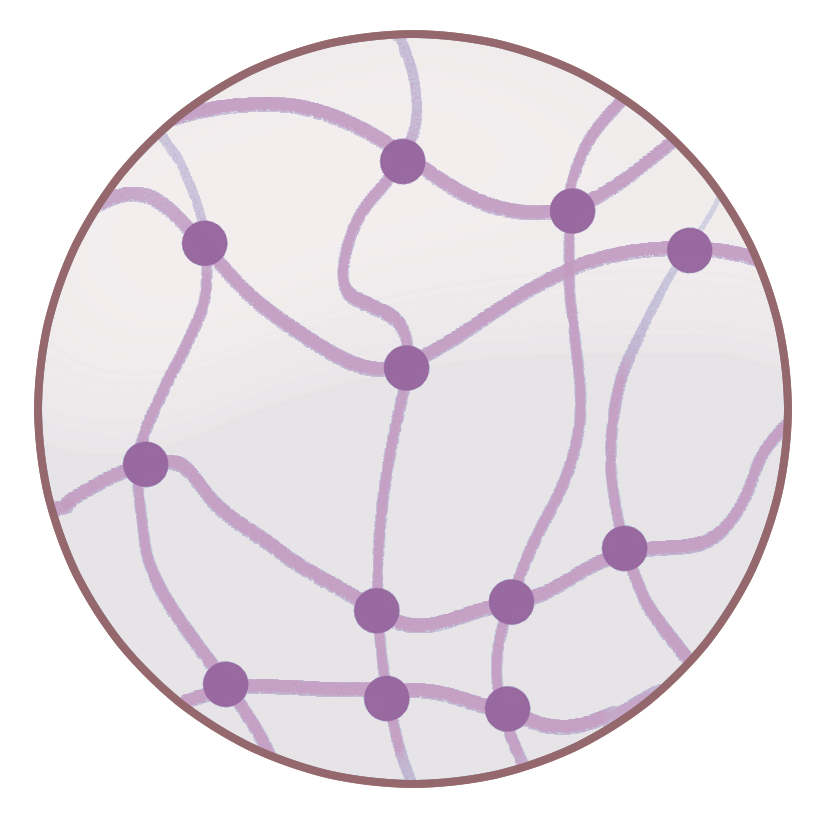
Figure 1 Hydrophilic cross-linked polymer network in hydrogel.
Poly(2-hydroxyethyl methacrylate) (pHEMA) was found suitable to make soft hydrogel lenses due to its higher oxygen transmissibility [4]. To further enhance the oxygen transmissibility, co-polymers were created by adding other monomers to the hydrogel mix to modify the properties of the material. But still, the lenses could not be worn for extended periods [4].
Further efforts led to the development of silicone hydrogel contact lenses [4]. Silicones, which have an even higher oxygen transmissibility than water, are polymers that contain silicon and oxygen. The use of silicone hydrogel enabled the invention of continuous-wear contact lenses, which can be worn overnight. However, silicone is a hydrophobic (water-repelling or lipid-loving) material so it is prone to problems like sticking to the eye surface which is covered by the tear film lipid layer [8]. After a few attempts to increase the hydrophilicity, bioengineers eventually solved the problem also by modifying the polymer.
Contact Lens Solutions
Contact lens solutions play a vital role in maintaining the cleanliness and functionality of contact lenses. They are specifically designed to disinfect, clean, and store the lenses, ensuring optimal comfort and vision for wearers. These solutions contain various chemical compounds that serve specific purposes.
There are two main types of contact lens cleaning solutions: peroxide solutions and multi-purpose solutions [4, 9]. They differ by the way they disinfect. Peroxide solutions utilize hydrogen peroxide (H2O2) as a disinfectant, typically at a 3% concentration. To make them safe for the eyes when lenses are reinserted after disinfection, a neutralization catalyst, such as platinum, palladium, or silver, present in the contact lens case is used to speed up the degradation of peroxide into water and oxygen.
Multi-purpose solutions commonly contain disinfection agents such as polyhexamethylene biguanide or polyquaternium-1 [4, 9]. These polymers possess optimal antimicrobial properties, and are derived from monomers with stronger antimicrobial activity but too harsh to be applied to the eyes before polymerization. It is speculated that polyhexamethylene biguanide works by selectively binding and condensing bacterial DNA, eventually blocking cell division [10, 11]. Polyquaternium-1 was found to cause leakage of cellular contents in ocular pathogens by disrupting the cell membrane [12, 13].
In addition to disinfection, contact lens solutions also include other compounds to maintain lens cleanliness and performance [4, 9]. Bisphosphonates help break down proteins that accumulate on the lens during wear, while moisturizing and conditioning chemicals ensure that the lenses remain in good condition while stored. There are also buffers to keep the pH gentle on the eyes, and preservatives to increase shelf life.
Tried-and-True Techniques & Tips for Beginners
For those who are new to contact lenses, the prospect of putting them on and taking them off can be quite intimidating. The thought of touching your eye or inserting a foreign object into it may seem daunting, but fear not! There are techniques that can make this process easier and potentially less frightening.
Since most contact lenses sold today are hydrophilic, you should use a dry hand to put on contact lenses so that the lenses will not stick to your hand. When you remove contact lenses, using a wet hand will make the task easier, but always make sure you wash your hands thoroughly before handling contact lenses.
It is advised not to use eyedrops when wearing contact lenses because that may cause problems with the lenses [14]. Nevertheless, you may consider wetting drops or preservative-free lubricating drops approved by your eye doctor if you often feel dry with contact lenses on. However, never use peroxide solution as wetting eyedrops – eyedrops and contact solution are not the same!
Conclusion
Contact lenses are a great alternative to glasses for those who feel bothered by the inconvenience and discomfort of wearing glasses. There are also lenses of different colors and graphic diameters for people who want to change their eye appearance. By understanding the different types of contact lenses available and how to wear them properly, you can make an informed decision about which contact lenses are right for you.
References
[1] Department of Health. (2022, June). Myopia. Student Health Service. https://www.studenthealth.gov.hk/english/health/health_ev/health_ev_nea.html
[2] Novak, J. F., & Saul, R. W. (1971). Contact Lenses in Industry. Journal of Occupational Medicine, 13(4), 175-178.
[3] Schifrin, L. G., & Rich, W. J. (1984, December). The Contact Lens Industry: Structure, Competition, and Public Policy. U.S. Congress, Office of Technology Assessment. https://www.princeton.edu/~ota/disk3/1984/8409/8409.PDF
[4] Brunning, A. (2015, October 13). The Chemistry of Contact Lenses. Compound Interest. https://www.compoundchem.com/2015/10/13/contactlenses/
[5] Wu, S. (2021). A Brief Review of Contact Lens Development in China. Boli tangci yu yanjing [Glass, Enamel and Eyeglasses], 49(7), 41-44. https://doi.org/10.13588/j.cnki.g.e.2096-7608.2021.07.007
[6] Lee, S. E., Kim, S. R., & Park, M. (2015). Oxygen permeability of soft contact lenses in different pH, osmolality and buffering solution. International Journal of Ophthalmology, 8(5), 1037-1042. https://doi.org/10.3980/j.issn.2222-3959.2015.05.33
[7] Alipour, F., Khaheshi, S., Soleimanzadeh, M., Heidarzadeh, S., & Heydarzadeh, S. (2017). Contact Lens-related Complications: A Review. Journal of Ophthalmic & Vision Research, 12(2), 193-204.
[8] Cwiklik, L. (2016, October). Tear film lipid layer: A molecular level view. Biochimica et biophysica Acta, 1858(10), 2421-2430. https://doi.org/10.1016/j.bbamem.2016.02.020
[9] Kemsley, J. (2008, November 7). What's that stuff? Contact Lens Solutions. Chemical & Engineering News, 86(46). https://cen.acs.org/articles/86/i46/Contact-Lens-Solutions.html
[10] Worsley, A., Vassileva, K., Tsui, J., Song, W., & Good, L. (2019). Polyhexamethylene Biguanide:Polyurethane Blend Nanofibrous Membranes for Wound Infection Control. Polymers, 11(5), 915. https://doi.org/10.3390/polym11050915
[11] Sowlati-Hashjin, S., Carbone, P., & Karttunen, M. (2020). Insights into the Polyhexamethylene Biguanide (PHMB) Mechanism of Action on Bacterial Membrane and DNA: A Molecular Dynamics Study. The Journal of Physical Chemistry B, 124(22), 4487–4497. https://doi.org/10.1021/acs.jpcb.0c02609
[12] Codling, C. E., Maillard, J., & Russell, A. D. (2003). Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine. The Journal of Antimicrobial Chemotherapy, 51(5), 1153–1158. https://doi.org/10.1093/jac/dkg228
[13] Codling, C. E., Hann, A. C., Maillard, J., & Russell, A. D. (2005). An investigation into the antimicrobial mechanisms of action of two contact lens biocides using electron microscopy. Contact Lens & Anterior Eye, 28(4), 163–168. https://doi.org/10.1016/j.clae.2005.08.002
[14] Boyd, K. (2022, April 22). How to Take Care of Contact Lenses. EyeSmart, American Academy of Ophthalmology. https://www.aao.org/eye-health/glasses-contacts/contact-lens-care