Artificial Leaves: The Door to an Infinite Supply of Green Power and Clean Air
By Alissa Lui 呂一蘭

Ninety-five percent of the global population is breathing in polluted air as of today [1], and within the last 200 years, 2.3 trillion tons of carbon dioxide has been released into our atmosphere [2]. These are two of the many figures showing the environmental damage humans have caused. Scientists and environmentalists have been on a quest to find a practical solution to reduce greenhouse gas emissions. One of the possibilities is the creation of the artificial leaf – a renewable power station no bigger than your hand.
The artificial leaf takes advantage of artificial photosynthesis, which converts carbon dioxide and water to energy-dense fuels, under the supply of solar energy. The whole process can be divided into two main parts, splitting of water and generation of energy-dense fuels.
The water-splitting reaction takes place in a photoelectrochemical cell (PEC) inside the artificial leaf. In the PEC, photoelectrodes that are composed of catalysts are submerged in water while absorbing light energy to split water into oxygen and hydrogen [3,4] (Figure 1). The water-splitting reaction involves the half reactions below:
Reduction at the cathode:
2H+ (aq) + 2e− → H2 (g)
Oxidation at the anode:
2H2O (l) → O2 (g) + 4H+ (aq) + 4e–
Overall equation:
2H2O (l) → 2H2 (g) + O2 (g)
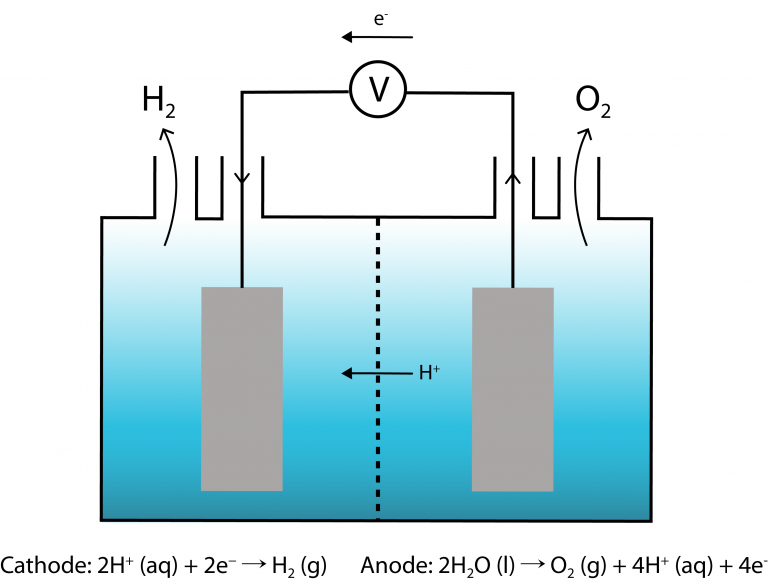 |
| Figure 1. |
The membrane in the PEC keeps both half-reactions separated, while allowing ions to pass through. This prevents charge imbalance in either half-cell, which could eventually lower the efficiency of the PEC. From a safety point of view, the membrane also keeps the products of both half-reactions, i.e. hydrogen and oxygen, separated to prevent explosion.
For the generation of energy-dense fuels, hydrogen and carbon dioxide can be utilised to produce hydrocarbon fuels with the help of a bioengineered bacterium, Ralstonia eutropha. Hydrogen (resulting from the photolysis of water) and carbon dioxide (from the atmosphere) can be consumed by the transgenic bacteria engineered by Nocera et al. to produce liquid fuels [4,5]. Prof Nocera from Harvard University reports that his system can still operate at low temperatures and costs, while maintaining its efficiency [3].
Additionally, it is worth noting that powering a home each day only requires a few litres of water. Prof Nocera and his colleague, Prof Silver, have devised a plan to produce liquid fuels at a 10% efficiency, a leap from the natural photosynthetic rate of (at most) 1% [3].
Scientists have been searching for the best choice of catalysts for the water-splitting step in the photochemical cell in the past few years. The potential consequence of corrosion and dissolution in the working environment rules out most of the economical catalysts. As you may have noticed above, the oxidation reaction turns the water near anode acidic, while the reduction reaction turns the water near cathode basic. This may be unfavourable to some cheap catalysts. However, Prof Lewis from the California Institute of Technology and his collaborators discovered that a coating of titanium dioxide can provide photoelectrodes and catalysts some protection in basic environments [4]. This discovery can potentially reduce the cost of a photoelectrochemical cell because an expensive catalyst is not required in cathode anymore. Nevertheless, a more affordable catalyst is yet to be found for the anode.
The use of the bioengineered bacteria R. eutropha in fuel production presented additional obstacle to the choice of catalysts for water splitting. To this end, Prof Nocera and Prof Silver have experimented with different materials, and the first was a nickel-molybdenum-zinc alloy [6]. Despite its high efficiency of water splitting, reactive oxygen compound byproducts were generated, which destroyed the bacteria’s DNA. In response to this adverse effect, some argued that bacteria should be replaced by inanimate catalytic materials for the step of fuel production, so that the whole system does not involve living organism. Systems composed entirely of inorganic material can usually work in a more stable manner, as living organisms are usually more sensitive towards temperature and pH fluctuations [4]. While it is still arguable whether transgenic bacteria is the best choice in this case, in 2016, Nocera et al. have shown that a novel cobalt-phosphorus alloy, with self-assembling properties, can be used as catalyst in water splitting without producing compounds that harm the bacteria [4,6]. The system constructed by the combination of the biocompatible catalyst along with the bacteria can result in an overall efficiency as much as 10% [4].
Despite the similarities found between artificial leaves and photovoltaics (also known as solar panels), artificial leaves will be more cost-effective and efficient, while providing us with cleaner air [7]. In the near future, roofs across the world could be embedded with artificial leaves, providing our homes with fresh air and electricity in a sustainable way. Who knew that the combination of synthetic biology and chemistry could revolutionise our society to this extent?
References
[1] Harvey, Fiona. “More than 95% of World’s Population Breathe Dangerous Air, Major Study Finds.” The Guardian, 17 Apr. 2018, www.theguardian.com/environment/2018/apr/17/more-than-95-of-worlds-population-breathe-dangerous-air-major-study-finds.
[2] The World Counts. “Facts About Environmental Issues.” http://www.theworldcounts.com/stories/Facts-About-Environmental-Issues.
[3] Martin, Richard. “This Bionic Leaf Is Better at Photosynthesis than a Real Leaf.” MIT Technology Review, 29 Jan. 2018, http://www.technologyreview.com/s/601641/a-big-leap-for-an-artificial-leaf/.
[4] Sivaram, Varun. “The Race to Invent the Artificial Leaf.” MIT Technology Review, 23 Feb. 2018, http://www.technologyreview.com/s/610177/the-race-to-invent-the-artificial-leaf/.
[5] Liu, Chong, Colón, Bredan C, Ziesack, Marika, Silver, Pamela A, and Nocera Daniel G. “Water splitting-biosynthetic system with CO₂ reduction efficiencies exceeding photosynthesis.” Science. 3 Jun. 2016;352(6290):1210-3. doi:10.1126/science.aaf5039
[6] Reuell, Peter. “Bionic Leaf Turns Sunlight into Liquid Fuel.” Harvard Gazette, 18 Dec. 2017, http://news.harvard.edu/gazette/story/2016/06/bionic-leaf-turns-sunlight-into-liquid-fuel/.
[7] Chandler, David L. “’Artificial Leaf’ Makes Fuel from Sunlight.” MIT News, 30 Sept. 2011, http://news.mit.edu/2011/artificial-leaf-0930.